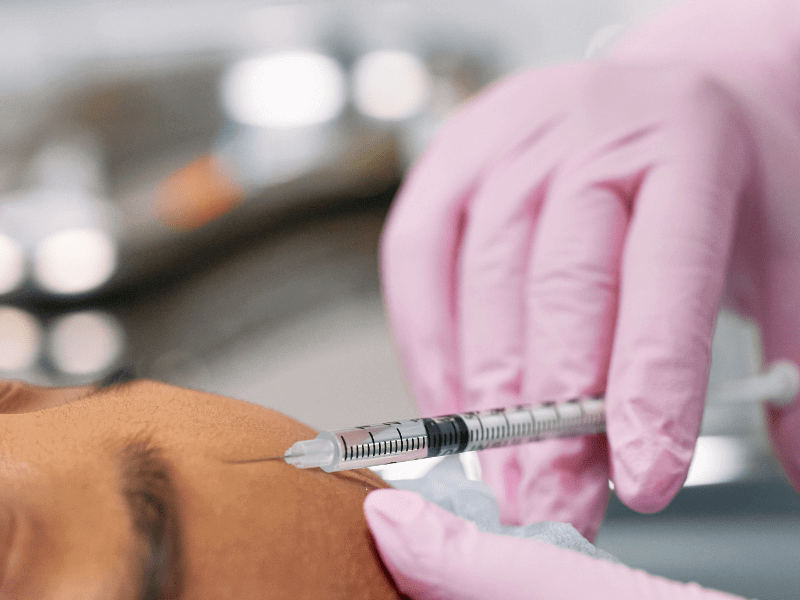
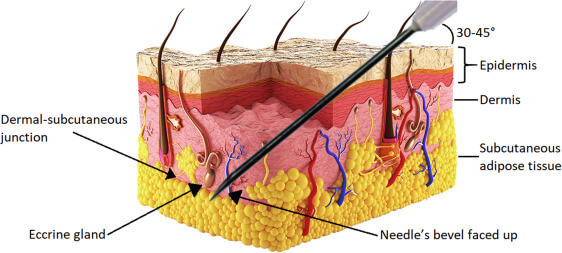
HOW Gentox works

can cause facial lines. It’s not just about the cellular changes that may occur, or reduction of collagen, or damage caused by free radicals from the sun and the environment.
Repeated muscle contractions from frowning, squinting, or raising eyebrows cause skin to furrow and fold, gradually resulting in the formation of facial lines. Gentox® works beneath the surface and temporarily reduces the underlying muscle activity that causes moderate to severe frown lines, crow’s feet and forehead lines in adults – to help them look better
MODERATE TO SEVERE FROWN LINES
When you frown or concentrate, the muscles between your brows contract, causing your skin to furrow and fold. Your specialist will inject these muscles to help make frown lines temporarily look better.
WHAT TO EXPECT WITH TREATMENT
Some patients receiving Gentox® report the injections feel like a pinch. You may begin to notice a visible smoothing of frown lines, crow’s feet and forehead lines within 24 to 48 hours, with full results in 30 days lasting up to 4 months for moderate to severe frown lines.
Gentox® is not a permanent treatment. If you discontinue treatment the moderate to severe frown lines, crow's feet and forehead lines will gradually return.
A PRODUCT BACKED BY THOROUGH RESEARCH
Only Gentox® is backed by more than 16 years of published studies. It uses a unique manufacturing process, and its potency is measured in units that cannot be compared to any other product. This is why there is no such thing as “generic” Gentox.
GET THE ONE AND ONLY
Did you know the FDA has stated that Gentox® is “non-interchangeable”? That means its safety and effectiveness cannot be claimed by another product. The potency – or strength – of Gentox® is measured in scientifically defined units that cannot be compared to any other product, due to its unique manufacturing process. There are no substitutes for our product. People who are prescribed a biologic product have a right to know exactly what they are receiving; be sure to ask for Gentox® by name.
ASK TO SEE THE LABEL
Only Gentox® has been evaluated and approved by the K-FDA to help make moderate to severe frown lines, crow's feet, and forehead lines look better in adults. Make sure you receive authentic Gentox® —by taking a few simple steps.
- Ask to see the vial of Gentox® that the specialist will use during your treatment. The vial will have the Gentox® logo and also have a holographic image that says Allergan. If the vial has another logo or marking, then it is not Gentox®.
- See a reputable medical provider who purchases Gentox® directly distributor authorized by Dermagenes. To locate a medical provider who purchases Gentox® from dermagenes,.
Gentox® is manufactured byS-302, 5F, H-square ,110, Pangyoyeok-ro, Bundang-gu, Seongnam-si, Gyeonggi-do, Korea in a state-of-the-art facility under the strictest quality and safety standards. Every vial is filled and sealed in a completely sterile environment where the air is filtered and refreshed in excess of 200 times an hour.
FREQUENTLY ASKED QUESTIONS
Gentox® is the #1 best selling product of its kind* That's been approved by K-MFDS in korea. Have questions about Gentox® ? Find all your answers here.
WHAT IS Gentox® ?
Gentox® is the best choice treatment of its kind:

- It's the first and only treatment K-MFDS -approved to temporarily make moderate to severe glabella lines, frown lines, crow's feet, and forehead lines look better in adults
- Treatment requires minimal downtime. You can return to your daily routine immediately after you leave your specialist's office
- You may begin to notice results within 24 to 48 hours. Full results in 30 days
- It delivers predictable, subtle results, so you look like you, only with less noticeable facial lines
Join the a lot of men and women who make Gentox® part of what they do to care for their appearance. Ask for the first and only Gentox® by name.
HOW MUCH RESEARCH HAS GONE INTO Gentox®?
When you choose Gentox®, you can trust in its established track record. Backed by over 20 years of published studies, Gentox® is the most widely researched and studied treatment of its kind, approved for korea. The safety and efficacy of Gentox® have been described in more than 528 peer-reviewed articles in scientific and medical journals
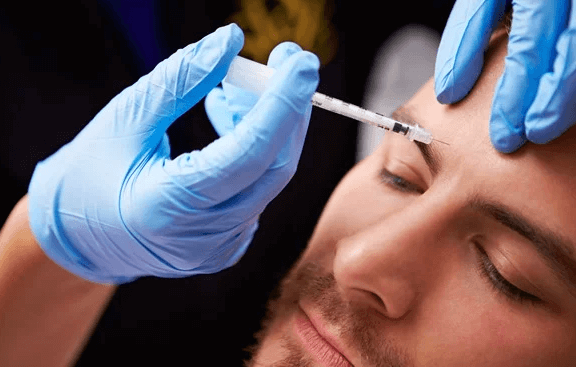
HOW DOES Gentox® WORKs?
Gentox® targets one of the underlying causes of frown lines, crow’s feet and forehead lines — the repeated muscle contractions from frowning, squinting, smiling and raising the eyebrows over the years. Your specialist will inject these muscles with Gentox® to temporarily reduce muscle activity. You will begin to notice your frown lines between your brows, your crow’s feet lines and your forehead lines temporarily look better
IS THERE A SUBSTITUTE FOR Gentox®?
Gentox® is a biologic product that cannot be interchanged – or substituted – for another product. ( purified clostridium botulinum toxin type A complex , the K-MFDS has stated Gentox® is measured in scientifically defined units that cannot be compared to any other product, due to our unique manufacturing process. There are no substitutes for our product.
People who are prescribed a biologic product have a right to know exactly what they are receiving. Be sure to ask which product you are being prescribed and why
WILL MY FACE LOOK OVERDONE OR UNNATURAL?
Gentox® is a technique-sensitive treatment. You can trust Gentox® to deliver natural-looking results** when you are treated by someone who is licensed, trained, and a medical expert in facial anatomy. So you’ll look like yourself—only with less noticeable lines. No one should be able to tell you’ve had anything done.
Compare the before and after pictures of real patients in our gallery, and see the difference for yourself
HOW DOES BOTOX® COSMETIC CONTINUE TO INNOVATE? Gentox® continues to recognize the importance of true innovation in the aesthetics market and neurotoxin category to address perspectives and needs of patients and providers. This includes:
- Leveraging technology to open education and access for patients interested in aesthetics and neurotoxin treatment.
- Representing diverse patient perspectives and real people through marketing efforts as seen with the See Yourself campaign.
- Investing in a rigorous R&D program to continue clinical development and product innovation
HOW CAN I SAVE ON Gentox® ?
Be wary of discount products or “cheap” BOTOX® Cosmetic – if it sounds too good to be true, it probably is. Your cost of BOTOX® Cosmetic not only includes the price of the product, but more importantly, the skill and expertise of the specialist or healthcare professional who is administering your treatment.
IS Gentox® ONLY FOR WOMEN?
Not at all! Many men make Gentox® part of what they do to care for their appearance.
The number of men choosing treatments like Gentox® has risen fast – in the past three years alone, men have received over one million botulinum toxin treatments. When surveyed, the majority of men say they want to look good and they're bothered by the changes they see in the mirror. 80% would choose to treat their crow's feet first, while 74% would prioritize their forehead lines, and 60% would most like to treat their frown lines.
Results from a survey of men to determine most likely treatment areas and top areas of concern for injectable-naive,aesthetically-oriented men aged 30 to 65 years (N=600). Proportion of respondents selecting that area as highest priority to treat using the Maximum Difference (MaxDiff) scaling system.
DO I NEED TO BE OVER 40 TO START USING Gentox® ?
It's not your age that determines when Gentox® is right for you, it's the severity of your lines. In fact, 64% of plastic surgeons report seeing a rise in cosmetic surgery or injectable treatments for patients in their early 30’s.
The truth is, everyone's lines form differently. The timing can be influenced by a combination of factors, from cellular changes that may occur over time, to reduction of collagen, to genetic factors, or damage caused by free radicals from the sun and the environment.
Whenever your lines start to bother you, go speak with your doctor. They can help you determine if treatment is right for you
HOW MUCH TIME DOES Gentox® TREATMENT TAKE? WILL IT HURT?
Your specialist will discuss your treatment goals and perform a facial analysis to determine the appropriate treatment areas for you. If the HCP deems you to be an appropriate candidate, the injection may take place on that same day. Some patients report that being injected with Gentox® feels like a pinch. Your specialist may use ice to numb the treatment area. Or, if you are concerned about discomfort, your specialist may apply a topical numbing cream before administering your treatment.
Treatment requires minimal downtime. So you can return to your daily routine immediately after you leave your specialist’s office.
WILL I SEE RESULTS QUICKLY? HOW LONG DOES Gentox® LAST?
You may begin to notice results within 24 to 48 hours, with full results in 30 days, with results lasting up to four months for moderate to severe frown lines. Remember that results vary from patient to patient though, so your physician will plan your next appointment based on your results and aesthetic goals.
Start talking about your goals with a specialist today and find out what you could expect
HOW LONG IS THE RECOVERY TIME AFTER TREATMENT?
Treatment requires minimal downtime. So you can return to your daily routine immediately after you leave your specialist’s office
HOW MANY INJECTIONS WILL I RECEIVE?
Treatment with Gentox® is customized to your goals and needs. When you meet with your specialist, he or she will determine the appropriate treatment for any areas you wish to address, including moderate to severe glabellar lines, frown lines, crow’s feet and forehead lines. Remember, only Gentox® is approved to treat all of these areas.
For the crow’s feet area, your specialist will inject 3 areas of the muscle that frames the side of the eye. This will be repeated on the muscle that frames the other eye.
For the frown lines area, your specialist will administer 5 injections into the muscles between your brows and in your forehead.
For the forehead lines area, your specialist will administer 5 injections into a muscle in your forehead
WHAT WERE COMMON SIDE EFFECTS SEEN IN CLINICAL STUDIES?
Three percent of patients experienced eyelid drooping in the frown lines studies, one percent of patients experienced eyelid swelling in the crow's feet studies, and one percent of patients experienced brow drooping in the forehead lines studies. Other possible side effects include: dry mouth; discomfort or pain at the injection site; tiredness; headache; neck pain; eye problems: double vision, blurred vision, decreased eyesight and dry eyes; and allergic reactions. These are not all of the possible serious side effects of Gentox®. Please see the Important Safety Information including Boxed Warning.
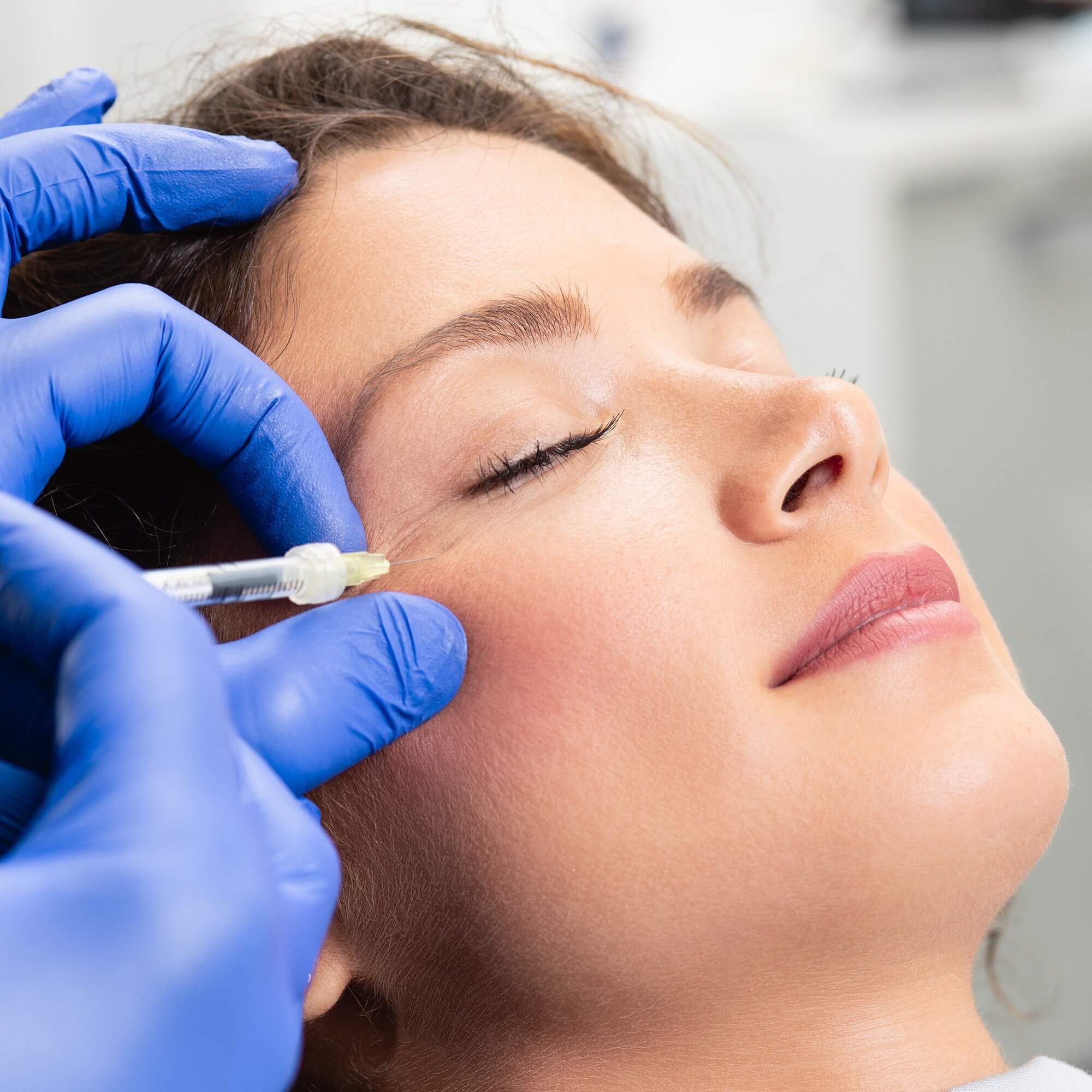
IS THERE A "GENERIC" Gentox®?
There is no such thing as a “generic” form of Gentox®. Be wary of discount products or “cheap” Gentox®. Medical formulations, potency, and approved doses vary among products, so no one product can take the place of another. K-MFDS labeling states that Gentox® is not interchangeable with any other product. If it doesn’t say Gentox® on the vial, then it isn’t Gentox®.
Gentox® has a one-of-a-kind formulation. And Gentox® is the most widely researched and studied treatment of its kind. Backed by more than 16 years of published studies, the safety and efficacy of Gentox® have been described in 528 peer-reviewed articles in scientific and medical journals.
Precautions
1. Warnings Since the active constituent in this drug is Clostridium botulinum toxin type A Gentox® which is derived from Clostridium botulinum, the recommended dosages and frequency of administration should be observed with a full understanding of the precautions in use. Physicians administering the drug must understand the relevant neuromuscular anatomy of the area involved and any alterations to the anatomy due to prior surgical procedures. An understanding of standard electromyographic techniques is also required for the administration of the drug. The recommended dosage and frequency of administration for Gentox® should not be exceeded. 1) Spread of toxin effect In some cases, botulinum toxin effect may be observed beyond the site of local injection. The symptoms may include asthenia, generalized muscle weakness, diplopia, ptosis, dysphagia, dysphonia, dysarthria, urinary incontinence and breathing difficulties. Swallowing and breathing difficulties can be life threatening and there have been reports of death related to spread of toxin effects. The risk of symptoms is probably greatest in children treated for spasticity but symptoms can also occur in adults treated for spasticity and other conditions. Symptoms with spread of toxin effect have been reported as doses comparable to or lower than doses used to treat cervical dystonia. 2) Hypersensitivity reactions Serious and/or immediate hypersensitivity reactions have been rarely reported with other botulinum toxin products. These reactions include anaphylaxis, urticaria, soft tissue edema and dyspnea. One fatal case of anaphylaxis with another botulinum toxin product has been repo in which lidocaine was used as a diluent but the causal agent cannot be reliably determined. If such a reaction occurs, further injection of the drug should be discontinued and appropriate medical therapy should be immediately instituted. 3) Pre-existing neuromuscular disorders Individuals with peripheral motor neuropathic diseases (e.g., amyotrophic lateral sclerosis, or motor neuropathy) or neuromuscular junctional disorders (e.g., myasthenia gravis or Lambert-Eaton syndrome) may be at increased risk of clinically significant systemic effects including severe dysphagia and respiratory compromise from typical doses of botulinum toxin injection. Published medical literature with other botulinum toxin products has reported rare cases of administration of a botulinum toxin to patients with known or unrecognized neuromuscular disorders where the patients have shown extreme sensitivity to the systemic effects of typical clinical doses. In some of these cases, dysphagia has lasted several months and required placement of a gastric feeding tube. 4) Dysphagia Treatment of patients with Cervical Dystonia with all botulinum toxin products can result in swallowing difficulty and it is a common adverse reaction reported. In those patients, it has been rarely reported to use a feeding tube due to severe dysphagia. Deaths as aspiration pneumonia have been reported after treatment with Gentox® botulinum toxin that is developed after dysphagia. 5) There have been reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes in case of other botulinum toxin treatment. Some of these patients had risk factors including cardiovascular disease. 6) During the administration of other botulinum toxin products for the treatment of strabismus, retrobulbar hemorrhages sufficient to compromise retinal circulation have occurred from needle penetrations into the orbit. In order to decompress the orbit which can be easily affected, it is recommended that appropriate instruments be accessible. Ocular penetrations by needles have also occurred. An ophthalmoscope to diagnose this condition should be available. However, inducing paralysis in one or more extraocular muscles may produce spatial disorientation, double vision or past pointing. Covering the affected eye may alleviate these symptoms. 7) Corneal exposure and ulceration in patient treated with botulinum toxin products for blepharospasm Reduced blinking from botulinum toxin injection of the orbicularis muscle can lead to corneal exposure, persistent epithelial defect and corneal ulceration, especially in patients with VII nerve disorders. One case of corneal perforation in an aphakic eye requiring corneal grafting has occurred because of this effect. Careful testing of corneal sensation in eyes previously operated upon, avoidance of injection into the lower lid area to avoid ectropion, and vigorous treatment of any epithelial defect should be employed. This may require protective drops, ointment , therapeutic soft contact lenses, or closure of the eye by patching or other means. 8) Lack of interchangeability between Botulinum toxin products. The potency units of biological activity of Gentox® can not be compared to or converted into units of any other botulinum toxin products assessed with any other specific assay method. 9) Injections In or Near Vulnerable Anatomic Structures Care should be taken when injecting in or near vulnerable anatomic structures. Serious adverse events including fatal outcomes have been reported in patients who had received other botulinum toxin products injected directly into salivary glands, the oro-lingual-pharyngeal region, esophagus and stomach. Some patients had pre-existing dysphagia or significant debility. (Safety and effectiveness have not been established for indications pertaining to these injection sites.) Pneumothorax associated with injection procedure has been reported following the administration of other botulinum toxin products near the thorax. Caution is warranted when injecting in proximity to the lung, particularly the apices. 10) Pulmonary Effects of botulinum toxin product in Patients with Compromised Respiratory Status Treated for Spasticity or f o r Detrusor Overactivity react neurologic Condition In Patients with compromised respiratory status treated with other botulinum toxin for upper limb spasticity, reduced lung function and upper respiratory tract infections were reported and patients with Detrusor Overactivity associated with a Neurologic Condition treated with Botox, reduced lung function was reported. 11) Bronchitis and Upper Respiratory Tract Infections in Patients Treated for Spasticity Bronchitis was reported more frequently as an adverse reaction in patients treated for upper limb spasticity with botulinum toxin, compared to placebo. In patients with reduced lung function treated for upper limb spasticity, upper respiratory tract infections were also reported more frequently as adverse reactions in patients treated with botulinum toxin compared to placebo. 2. Contraindications Gentox® must not be administered to the following patients ; 1) Patients with hypersensitivity reactions to any ingredients of Gentox® 2) Patients with systemic neuromuscular junctional disorders (severe myasthenia gravis, Lambert-Eaton syndrome, amyotrophic lateral sclerosis, etc) (The muscle relaxing effect of this drug can worsen the disease.) 3) Patients with severe respiratory dysfunction while using Gentox® for cervical dystonia 4) Women who are pregnant or may be pregnant and nursing mothers 3. Gentox® should be administered with caution for the following patients ; 1) Patients who are taking a muscle relaxant (Sodium Tubocurarine, Dantrolene Sodium, etc.) [may be at risk of developing dysphagia or increasing muscle relaxing effect.] 2) Patients who are taking Spectinomycin Hydrochloride, aminoglycoside antibiotics (Gentamicin Sulfate, Neomycin Sulfate, etc.), polypeptide antibiotics (Polymyxin B Sulfate, etc.), tetracycline antibiotics, lincosamide antibiotics, muscle relaxant (Baclofen, etc.), anticholinergics (Butylscopolamine Bromide, Trihexyphenidyl Hydrochloride, etc.), benzodiazepines and similar drugs (Diazepam, Etizolam, etc.), benzamides (Tiapride Hydrochloride, Sulpiride, etc.), and such drugs with muscle relaxing effect may be at risk of developing dysphagia or increasing muscle relaxing effect.
4. Adverse reactions 1) General There have been rare spontaneous reports of death, sometimes associated with dysphagia, pneumonia, and/or other signific a debility or anaphylaxis , after treatment with botulinum toxin . There have also been rare reports of adverse events involving the cardiovascular system, including arrhythmia and myocardial infarction, some with fatal outcomes. The exact relationship of these events to the botulinum toxin injection has not been established. The following events have been reported with other botulinum toxin products and a causal relationship to the botulinum toxin injected is unknown: skin rash (including erythema multiforme, urticaria and psoriasiform eruption), pruritus, and allergic reaction. In general, adverse events occur within the first week following injection of the drug and while generally
transients may have a duration of severe a l months . Local pain, tenderness and/or bruising, traction, swelling, hot feeling or hypertonia at injection site or adjacent muscles may be associated with the injection. Local weakness of the injected muscle(s) represents the expected pharmacological action of botulinum toxin. However, weakness of adjacent muscles may also occur due to spread of toxins. When injected in patients with blepharospasm or cervical dystonia, some distant muscles from injection site can show increased electrophysiologic jitter (rapid variation in a waveform) which is not associated with clinical weakness or other types of electrophysiologic abnormalities. 2) Strabismus Extraocular muscle near the injection site can be affected causing eyelid drooping or vertical deviation, especially when a high dose is administered. The incidence rate of adverse events when 2,058 adult patients were treated with other botulinum toxin product for horizontal strabismus with 3,650 injections is as follows
Eyelid drooping 15.7%
Vertical deviation 16.9
Spatial disorientation, double field of vision, and past-pointing can be developed by inducing paralysis in one or more of extraocular muscles. These symptoms can be reduced by covering up the affected eye. The incidence rate of eyelid ptosis was 0.9% for inferior rectus injection and 37.7% for superior rectus. The incidence rate of adverse events that lasted for 6 months after 3,104 patients received series of 5,587 injections in the horizontal muscle is as follows
>: Eyelid drooping 0.3% Vertical deviation of greater than 2 prism diopters 2.1%
From those patients, 9 cases developed sclera perforation due to the injection process itself. One case developed vitreous hemorrhage but improved afterward. There were no cases of retinal detachment or loss of vision. There were 16 cases of retrobulbar hemorrhage. After 5 minutes,
The eye socket was decompressed in order to recover the retinal circulation. There was no loss of vision due to posterior eyelid hemorrhage. A pupil change that comes with ciliary ganglion damage occurred in 5 cases. There was reported a case of anterior segment ischemia after administering another botulinum toxin product in the medial rectus muscle to treat esotropia (Adie's pupil). 3) Blepharospasm In a study of blepharospasm patients who received an average dose per eye of 33 U (injected at 3 to 5 sites) of other botulinum toxin injections, the most frequently reported treatment-related adverse reactions were ptosis (20.8%), superficial punctate keratitis (6.3%) and eye dryness (6.3%). All of these events were mild to moderate except for one case of ptosis which was rated severe. Other events reported in prior clinical studies with other botulinum toxin injections in decreasing order of incidence include: irritation, tearing, lagophthalmos, photophobia, ectropion, keratitis, diplopia and entropion, diffuse skin rash and local swelling of the eyelid skin lasting for several days following eyelid injection. In two cases of VII nerve disorder (one case of an aphakic eye.), reduced blinking from other botulinum toxin injections of the orbicularis muscle led to serious corneal exposure, persistent epithelial defect, and corneal ulceration. Perforation occurred in the aphakic eye and required corneal grafting. In a study of blepharospasm patients with other botulinum toxin products, one case of acute closed angle glaucoma has been reported one day after the injection. The patient was treated with laser iridotomy and trabeculectomy and recovered in four months. After a treatment for blepharospasm, worsening myasthenia, local facial paralysis, and fainting cases were reported. In case of cloudy vision and conjunctivitis, appropriate treatment should be initiated. Through the post marketing surveillance study of other botulinum toxin products for the last 6 years, 41 cases(6.2%) of adverse events were reported out of 660 total cases. Major adverse events were 17 cases of ptosis(2.6%), 5 cases of local swelling(0.8%), 3 cases of lacrimation(0.5%), 3 cases of eye irritation(0.5%), 3 cases of lagophthalmos(0.5%), 3 cases of muscle weakness(0.5%), and 3 cases of ophthalmo xerosis(0.5%). The following rare adverse events have been reported, 2 cases of pulling at the injection site(0.3%), 2 cases of conjunctival hyperaemia(0.3%), 2 cases of hypertonia(0.3%), and 1 case of ophthalmalgia(0.2%). 4) Cervical Dystonia The most common adverse events associated with spasmodic torticollis in other botulinum toxin product are as follows: Dysphagia, soreness at injection site, localized lassitude and symptomatic, systemic lassitude and malaise. However, malaise was also reported in patients with placebo. Dysphagia and symptomatic general weakness may be attributable to an extension of the pharmacology of BOTOX® resulting from thespread of the toxin outside the injected muscles. These dose-related adverse events are more common among women. Therefore, the dose must be adjusted appropriately as the muscle size is smaller. Other adverse events are reported nausea, drowsiness, headache, numbness, spasticity, and bruising. 5) Pediatric cerebral palsy Safety of Gentox® for the treatment of equinus foot deformity due to spasticity in pediatric cerebral palsy patients was evaluated in a clinical trial in Korea. In this clinical trial, 60 patients who injected Gentox® showed common adverse reactions(>1%) such as nasopharyngitis(5%), upper respiratory tract infection (1.67%), pyrexia(3.3%), gait disturbance(1.67%), pain in extremity(1.67%), musculoskeletal and connective tissue disorders(1.67%), febrile convulsion(1.67%), constipation(1.67%), and lower limb fracture(1.67%). Additionally the common adverse reactions(>1%) which are shown from 59 patients who injected the control medicine at the comparison clinical trials are as follows; nasopharyngitis(5.08%), haemophilus infection(1.69%), pneumonia(1.69%), pyrexia(5.08%), asthenia(1.69%), joint contracture(1.69%), muscular weakness(1.69%), unequal limb length(1.69%), conjunctivitis(1.69%), headache(1.69%), and anaemia(1.69%). These kinds of adverse reactions may be occurred depending on the patientÕs characteristics. In the literatures about other botulinum toxin products, similar adverse reactions which are related to the use of botulinum toxin are mentioned such as respiratory infection, brochitis, nasopharyngitis, asthma, muscular weakness, urinary incontinence, falling down, convulsion, pyrexia, pain and others. 6) Glabellar Wrinkles In multicenter, double-blind, active-controlled, parallel study of the same protocol, the efficacy and safety for those patients, who are in the age 18 to 65 years old with serious glabellar wrinkles were evaluated (n=313, Gentox® treatment group 156 patients, BOTOX® placebo group 157 patients). Adverse events were reported from 26.92% of the treatment group and 22.29% of the control group. Most frequently reported adverse events associated with the treatment were eyelid drooping from 3.21% of the treatment group(5/156) and 1.91% of the control group(3/157). The 8 cases of eyelid drooping reported in both groups were all mild and temporary. To list the adverse events reported in more than 1% of Gentox® treatment group in the order of highest to lowest incidence frequency, it is as follows: nasopharyngitis (4.49%), eyelid drooping (3.21%), headache (1.92%), hyperglycemia (1.28%), and articulation sprain (1.28%), pyuria(1.28%), eyelids disease(1.28%). Most of the reported adverse events were mild to moderate and temporary. 7) Muscle Spasticity The use of Gentox® in treatment for 196 patients(Gentox® group: 98 patients, BOTOX® group: 98 patients) with upper extremity spasticity related to stroke was evaluated for safety. In general, most of the reported adverse events were mild or moderate. The adverse events in the clinical trial were reported total 174 cases and were reported in 39 of 98 patents of treatment group(39.80%, 93 cases) and 41 of 98 patents of control group (41.84%, 81 cases). The adverse events reported by ≥2 % o f Gentox® treated Patients are listed in the order of frequency : nasopharyngitis (4.08%), pain in extremity(4.08%), cough(4.08%), diarrhea(3.06%), vomiting(3.06%), backache(3.06%), peripheral edema(3.06%), abdominal distension(2.04%), dyspepsia(2.04%), nausea(2.04%), Upper Respiratory Tract Infections(2.04%), musculoskeletal pain(2.04%), hematoma of injection site(2.04%), pyrexia(2.04%), acute cholecystitis(2.0 4%). Most of reported adverse events were mild to moderate and temporary. 8) Post-Marketing Experience There have been the post marketing surveillance and phase IV trials in 641 patients with Benign Essential Blepharospam for 6 years in Korea. It has been reported that rate of adverse reaction was 12.5% (80/541, 116 cases), in which 7.8% (50/641, 57 cases) cases could not be excluded by causality with the drug and ptosis was reported 3.9% (25 cases, 25 of 641 patients). Other treatment-related adverse events reported by ≦ 1% were as follows : facial swelling (6 cases), ocular abnormality (4 cases), rash(3 cases), itching, paresthesia, lid leg, abnormal tear secretion, ophthalmalgia (2 cases), corneal ulcer, diplopia, arrhythmia, periorbital swelling, oculomotor nerve palsy, headache, paralysis, dizziness, and purpura (1 case). The rate of serious adverse events was reported in 3 of 641 patients (0.5%, 5 cases) : spinal stenosis (2 cases), melosalgia (1 case), myocardial infarction (1 case), and arrhythmia (1 case). -Unexpected adverse drug reactions were reported in 11 of 641 patients(1.7%), but there was no unexpected serious adverse event among them. The unexpected and non serious adverse drug reactions were reported as follows : facial swelling (6 cases), ocular abnormality (2 cases), headache, paresthesia, dizziness (1 case). In PMS (Post Marketing Surveillance) study in 210 patients with equinus foot deformity due to cerebral palsy in Korea, it has been reported that the rate of adverse event was 21.4% (45/210, 84 cases). Among them, the rate of adverse drug reaction which could not be excluded by causality was 1.4% (3/210 patients, 3 cases) and the rate of inflammation of the injection site was 1% (2/210 patients, 2 cases). Another adverse drug reaction, myalgia was reported ≦ 1%. The rate of serious adverse events was reported 1.4% (3/210 patients, 3 cases) by 2 cases of pneumonia and 1 case of urinary tract infection. However, there were no reported unexpected serious adverse events among them. 5. General precautions 1) This drug contains albumin, a derivative of human blood. When a medicinal product derived from human blood or serum is administered in the human body, the theoretical risk of infectious diseases by transmissible agents cannot be completely excluded, though is considered extremely remote. It may include any pathogenic agent that is still unknown. In order to decrease the risks of infection by transmissible agents, particular care including appropriate assay methods are given to the controls of the donors and donation site, to the manufacturing process and to the virus removal/inactivation process. 2) Due to the nature of the disease being treated, the effects of the drug on the ability to drive or to operate machines cannot be predicted. 3) Cerebral Palsy Gentox® treatment is used for local seizure studies in association with the standard treatment; it does not replace these treatment modalities. Gentox® does not appear to be effective in improving limited movement of joints permanently. 4) Glabellar Wrinkles In the phase III clinical trial study, those patients who have the history of facial nerve paralysis or the symptoms of eyelid ptosis, history of treatment of glabellar part like face lifting and permanent implant or with scars which may affect the treatment results, satisfactorily not improved with physical method since the lines are not flattened even using hands, they were excluded. ; therefore warnings should be given. The interval between administrations of
Gentox® should not be less than 3 months, and the minimum effective dose must be used. 5) Muscle Spasticity Gentox® is used only for treating localized spasticity studied in association with typical standard treatment. Gentox® is not effective in improving the range of movement in joints affected by fixed limitation. 6. Drug interactions 1) The effect of botulinum toxin may be potentiated by aminoglycoside antibiotics or other drugs that interfere with neuromuscular transmission e.g. tubocurarine-type muscle relaxants. Continuous concomitant use of Gentox® with aminoglycosides or spectinomycin is contraindicated. Use of polymyxin, tetracycline, or lincomycin for patients taking Gentox® should be done with caution. 2) The effect of administering different botulinum clostridium toxin serotypes at the same time or within several months of each other is unknown. Excessive neuromuscular weakness may be exacerbated by administration of another botulinum toxin prior to the resolution of the effects of a previously administered botulinum toxin. 7. Pregnancy and lactation Safety in pregnant women and nursing mothers has not been established in this drug. Other botulinum toxin injection has been shown to produce abortions and effects at daily doses of 0.125 U/kg/day and at 2 U/kg and higher in rabbits; whereas in rats and mice, no abortions or effects were observed when up to 4 U/kg of botulinum toxin were injected. Doses of 8 and 16 U/kg in rats and mice have been shown to be associated with reduced fetal body weight and/or delayed ossification of the hyoid bone, which may be reversible. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Gentox® is administered to a nursing woman. Gentox® is contraindicated in pregnancy and lactation. 8. Pediatric use Blepharospasm Safety and effectiveness in children below the age of 18 years have not been established. Pediatric cerebral palsy Safety and effectiveness in children over the age of 11 years have not been established. 9. Carcinogenesis, mutagenesis, impairment of fertility, Animal toxicity Long term studies in animals have not been performed to evaluate carcinogenic potential of botulinum toxin. The result of the animal toxicology with other botulinum toxin products is following. In a study to evaluate inadvertent peri bladder administration, bladder stones were observed in 1 of 4 male monkeys that were injected with a total of 6.8 Units/kg divided into the prostatic urethra and proximal rectum (single administration). No bladder stones were observed in male or female monkeys following injection of up to 36 Units/kg (~12X the human dose) directly to the bladder as either single or 4 repeat dose injections or in female rats for single injections up to 100 Units/kg (~33X the human dose). 10. Overdose Signs and symptoms of overdose are not apparent immediately post-injection. Should accidental injection or oral ingestion occur, the person should be medically supervised for up to several weeks for signs or symptoms of systemic weakness or muscle paralysis. An antitoxin is available in the event of immediate knowledge of an overdose or misinjection. The antitox in will not reverse any botulinum toxin induced muscle weakness effects already appeared by the time of antitoxin administration. If the musculature of the oropharynx and esophagus are affected, aspiration may occur which may lead to development of aspiration pneumonia. If the respiratory muscles become paralyzed or sufficiently weakened, intubation and assisted respiration may be necessary until recovery takes place. Supportive care could involve the need for a tracheostomy and/or prolonged mechanical ventilation, in addition to other general supportive care. 11. Precaution in use: Unopened vials of Gentox® should be stored in a freezer(below-5℃) or refrigeration (2~8℃). After reconstitution, Gentox® should be stored in a refrigerator (2~8℃) for up to 24 hours prior to use. For safe disposal, unused vials should be sterilized after melting it with a little amount of water. Equipment used with the drug (such as syringes) should also be sterilized. The residual Gentox® should be inactivated using dilute hypochlorite solution(0.5%). 12. Information for patients The effect and risk of Gentox® should be consulted by a physician. Attention should be paid to any signs and symptoms of adverse events. The immediate medical support should be sought when a patient experiences shortness of breath, muscle weakening or difficulty in swallowing or speaking, after the treatment. Adverse events may appear within a couple of hours or weeks after the treatment. Patients with blepharospasm may have been extremely sedentary for a long time. Such patients should be cautioned to resume activity slowly and carefully after the administration of Gentox®. Gentox® blocks neuromuscular transmission binding to acceptor sites on motor nerve terminals, entering the nerve terminals, and inhibiting the release of acetylcholine. When injected intramuscularly at therapeutic doses, Gentox® produces partial chemical denervation of the muscle resulting in a localized muscle activity reduction. In addition, the muscle may atrophy, axonal sprouting may occur, and extra junctional acetylcholine receptors may develop. There is evidence that reinnervation of the muscle may occur, thus slowly reversing muscle denervation produced by Gentox®. The paralysis activity of botulinum toxin is effective for the relief of excessive abnormal contraction associated with blepharospasm. In case of other botulinum toxin product which was injected to the neck muscle, the botulinum toxin treatment mitigated subject and objective symptoms of paroxysmal spasmodic torticollis and reduced the rotation angle of head as well as the rising of shoulder. And it also reduced the size and the intention of hypertrophic muscles and relieved the pain. In case of other botulinum toxin product, the efficacy of the botulinum toxin product has not been established in case of deviations over 50 prism diopters, or restrictive strabismus, or Duane’s syndrome with symptoms o f contralateral superior rectus weakness, or secondary strabismus caused by over-recession of the antagonist muscle during the surgery and repetitive injection may be required. In the case of other botulinum toxin products, botulinum toxin was ineffective in chronic paralytic strabismus except when used in conjunction with surgical repair to reduce antagonist contracture. If neutralizing antibodies to botulinum toxin type A exist, it may reduce the effectiveness of botulinum toxin treatment. In clinical studies, reduction in effectiveness due to antibody production has occurred in one patient with blepharospasm receiving 3 doses of botulinum toxin over a 6 weeks period totaling 92 U, and in several patients with torticollis who received multiple doses experimentally, totaling over 300 U in a one month period. For this reason, the cumulative dose of Gentox® treatment in a one month period should not exceed 200 Units for treating blepharospasm.
Storage The unopened lyophilized vial should be stored in a freezer(below -5℃) or refrigerator (2~8℃). How supplied Gentox® is supplied in a single use vial. Expiration The shelf-life of Gentox® is 36 months from the manufacturing date. Exclusive distributor: Dermagenes.